A Next-Generation Nootropic From 11 years of Research and Development
An interdisciplinary neuroscience PhD student at the University of California found himself running out of hours in the day. Through high school and undergrad he’d managed without much chemical help, but the demands of his graduate work were on another level. Over the next five years, he tried everything-caffeine, Adderall, ginseng, racetams, choline, B vitamins, Lion’s Mane, creatine, even ice baths. Caffeine wrecked his sleep; Adderall made him grind his teeth. Nothing truly improved focus. Then he discovered phenylethylamine (PEA)-a compound the human the brain already produces naturally-that he found delivered an intense burst of clarity and motivation, but only for five minutes. Now leading his own lab, he teamed up with chemists and biochemists to design a stack that extended and amplified the state change offered by PEA. The result, Power Hour, offered pharmaceutical-grade focus from all-natural ingredients, without lingering side effects.
Phenylethylamine
PEA is a natural trace amine, derivative of the amino acid phenylalanine, it's released from the same neurons that produce alertness-promoting biogenic amines (such as dopamine and norepinephrine). It activates the Trace Amine-Associated Receptor (TAAR1), stimulating mesolimbic dopamine pathways (VTA → nucleus accumbens), associated with drive, reward, and reinforcement. In practice, this makes your brain feel rewarded for focusing on the task at hand.
This, incidentally, is the same trick played by amphetamine salts like Adderall, but as a compound that is already naturally synthesized and released in the brain, PEA can be metabolized more rapidly, making it safer.
The Stack
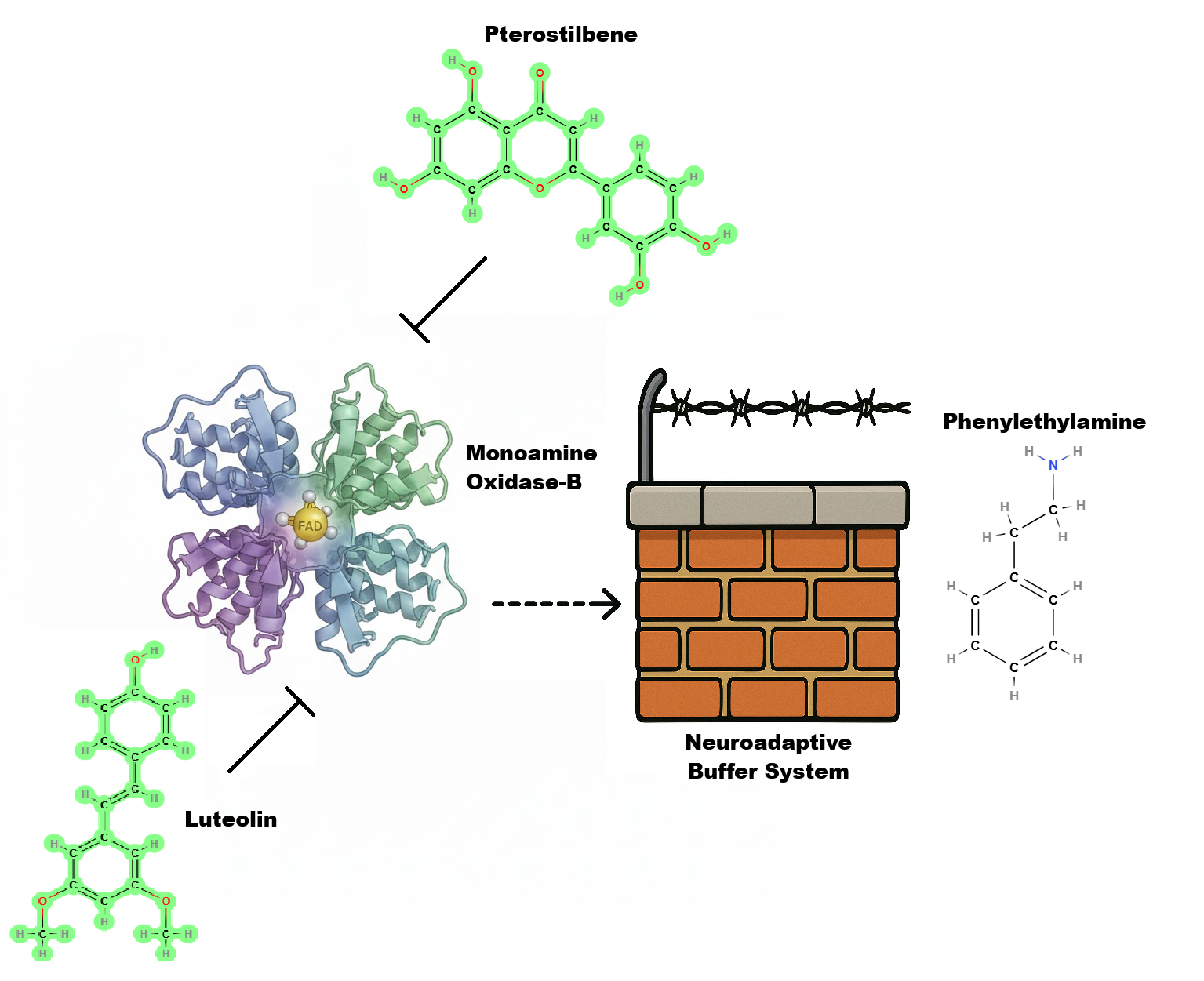
To extend PEA’s duration and smooth its neurochemical profile, the Power Hour Focus stack incorporates four key compounds: taurine, pterostilbene, luteolin, and our Neuroadaptive Buffer System™.
Taurine acts as a neuroprotective osmolyte and modulator of calcium homeostasis within neurons. It helps buffer excitatory activity associated with dopaminergic stimulation, promoting smooth and sustained neurotransmission without overstimulation or jitteriness. Taurine also supports mitochondrial energy metabolism, indirectly stabilizing PEA-driven catecholaminergic activity and preventing oxidative stress.
Pterostilbene, a methylated analog of resveratrol, functions as a potent antioxidant and MAO-B modulator. By attenuating the rapid enzymatic breakdown of PEA, pterostilbene prolongs its availability and functional window at TAAR1 receptors. Additionally, pterostilbene enhances neuronal resilience through upregulation of antioxidant response elements (via Nrf2 signaling) and may support cerebral blood flow, further amplifying cognitive performance.
Luteolin, a flavonoid found in many botanicals, provides complementary anti-inflammatory and MAO-B–inhibitory activity. It reduces neuroinflammatory signaling through NF-κB suppression and helps preserve dopaminergic neurons from reactive oxygen species generated during heightened neurotransmitter turnover. By subtly inhibiting MAO-B, luteolin synergizes with pterostilbene to extend the functional lifetime of PEA while maintaining a safe and physiologically balanced dopaminergic tone.
Neuroadaptive Buffer System, a proprietary mixture of natural, synergistic compounds. These three additional compounds have shown in trials to triple the duration of PEA in preliminary development trials. Though minimal in weight, this system is a critical component of our blend.